PRL-02 (abiraterone decanoate, intramuscular injection)
Surgical and chemical castration (e.g., Lupron Depot®, leuprolide acetate for depot suspension) are standards of care for metastatic prostate cancer (PC) but androgens produced in the adrenals, prostate, and the tumor itself can continue to drive tumor growth. The treatment goal for advanced disease is to block androgen receptor (AR) activation by these extra-gonadal androgens (e.g., testosterone). Current treatments for advanced or high-risk PC are suboptimal due to limited efficacy, safety, or compliance. Though several AR antagonists are approved for the treatment of PC, only one product is marketed (Zytiga®, abiraterone acetate) that blocks the enzyme, CYP17, that produces androgens. However, the oral abiraterone acetate regimen is complex and has safety and efficacy concerns, leading to patient compliance concerns.
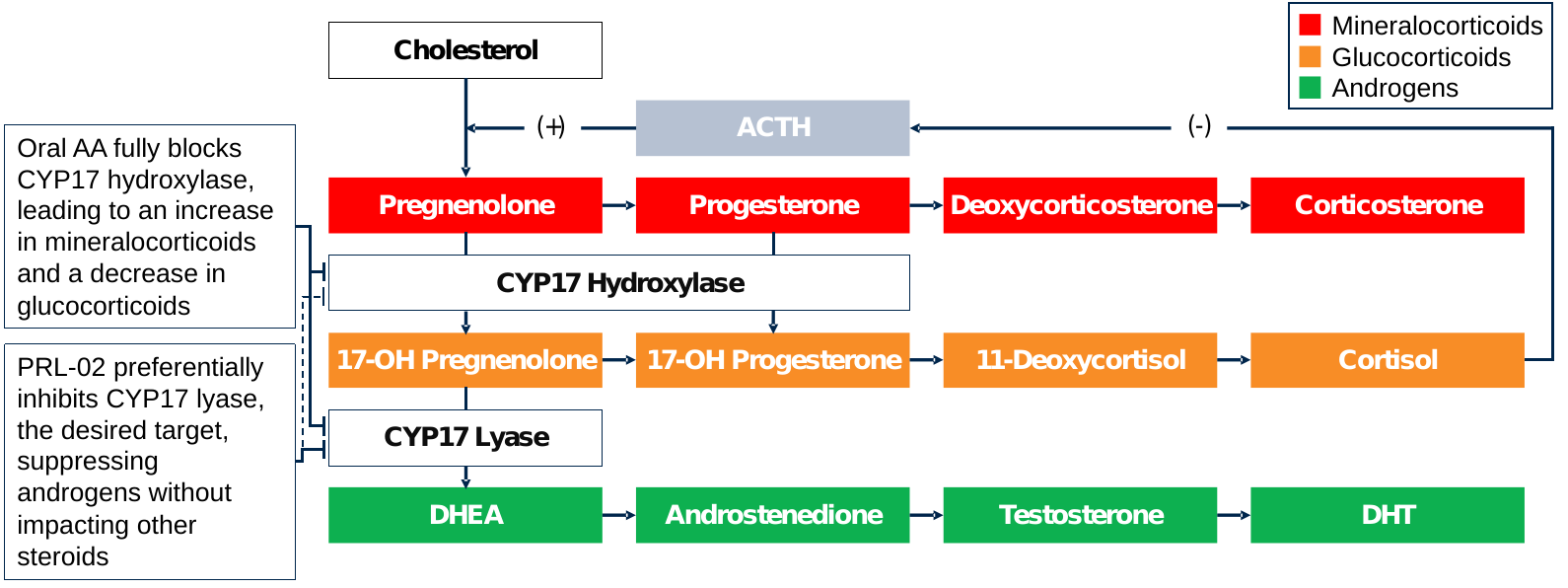
Moreover, the Zytiga® 1,000mg daily dose maximally inhibits CYP17 hydroxylase; however, CYP17 lyase is the preferred target enzyme activity (see the Figure below). Zytiga use is associated with efficacy and safety concerns that are respectively due to the elevation of upstream steroids (e.g., progesterone) and mineralocorticoids (e.g., corticosterone) due to CYP17 hydroxylase inhibition.
Emerging clinical data suggest that PRL-02 has a vastly improved efficacy and safety profile versus standard of care Zytiga®, while being significantly more convenient for patients. Efficacy and safety advantages versus Zytiga® are hypothesized to be due to no or minimal CYP17 hydroxylase inhibition, which can drive tumor resistance through the elevation of progesterone and pregnenolone and produces myriad safety signals specified on the product label through the elevation of mineralocorticoids, including hypertension and hypokalemia).
PRL-02 is currently in a Phase 1 dose-escalation study in patients with advanced PC and is expected to enter the Phase 2a portion of the study in the first half of 2025. Moreover, PRL-02 is demonstrating promising clinical benefit for men whose disease has worsened while being treated with standard of care androgen receptor antagonists including enzalutamide and apalutamide. The promising activity observed to date in second- and third-line prostate cancer patients is in sharp contrast to the results observed with Zytiga in these same patient populations. For further information, please see Propella’s presentation at the American Society of Clinical Oncology Genitourinary Symposium in San Francisco, CA on January 25, 2024. (click here).